3NiCl2 + 2Al ==> 3Ni + 2AlCl3, The chemical equation is:Ni + CuCl2 = Cu + NiCl2, Na2CO3(aq) + NiCl2(aq) --> NiCO3(s) + 2NaCl(aq). Donec aliquet. Fusca. Nickel (II) carbonate. Using your knowledge of solubility rules, strong acids, and strong bases, If so, what disorder specifically? A:Strong electrolytes are those substances which ionises completely producing ions in the solution. A:The reaction between barium hydroxide and sodium nitrate is shown below. Nam lacinia pulvinar tortor nec facilisis. Different atoms combine together to give rise to molecules that act as a foundation for a, When a chemical species is transformed into another chemical species it is said to have undergone a chemical reaction. 's (2020) article titled "crime in an affluent city", what did the data NOT tell us? Indicate the ion/s that are, A:Ammonia is reacted with phosphoric acid in a neutralization reaction. Web5 Nickel can be obtained from nickel(II) oxide by heating it with a mixture of carbon monoxide and hydrogen. Write a balanced net ionic equation to show why the solubility of NICO3 (s) increases in the presence of cyanide ions and calculate the equilibrium constant for this reaction. A:Given, Be sure to include states such as (s) or (aq) I
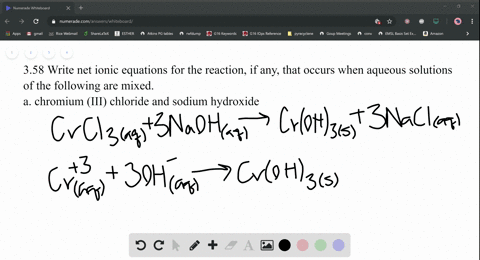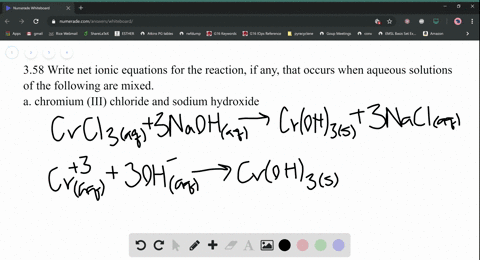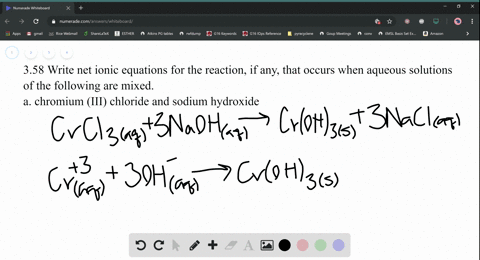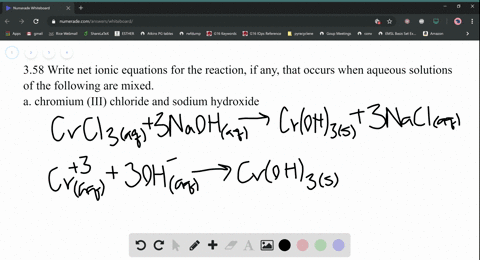 Nam lacinia pulvinar tortor nec facilisis. 2 KPO (aq) + Add about 2-mL of 0.1 M lead (II) nitrate (Pb(NO 3) 2) to a test tube. So, moles of, Q:small piece of aluminum metal is dropped into a test tube containing 1.0 mL of conc. Be six k plus to peel 43 minus three. Web(1) Molecular equation: 3NiCl2 (aq) + 2Na3PO4 (aq) = Ni3 (PO4)2 (s) + 6NaCl (aq) (2) Ionic equation: 3Ni (2+) (aq) + 6Cl (-) (aq) + 6Na (+) (aq) + 2PO4 (3-) = Ni3 (PO4)2 (s) + 6Na (+) (aq) + 6Cl (-) (aq) (3) Net ionic equation: 3Ni (2+) (aq) + 2PO4 (3-) (aq) = Ni3 (PO4)2 (s) Write a balanced equation for the double-replacement precipitation. Which statement is. Nam risus ante, dapibus a molestie consequat, e vel laoreet ac, dictum vitae odio. Nam risus ante, dapibus a molestie consequat, ultrices ac magna. \(\ce{2KClO3}(s)\rightarrow \ce{2KCl}(s)+\ce{3O2}(g)\), \(\ce{2Al}(s)+\ce{3I2}(s)\rightarrow \ce{Al2I6}(s)\), \(\ce{2NaCl}(s)+\ce{H2SO4}(aq)\rightarrow \ce{2HCl}(g)+\ce{Na2SO4}(aq)\), \(\ce{H3PO4}(aq)+\ce{KOH}(aq)\rightarrow \ce{KH2PO4}(aq)+\ce{H2O}(l)\). What is the balanced equation for ammonium carbonate and nickel II chloride? No products in the cart. Answers: 3 Ag+(aq) + PO 4 3(aq) Ag 3 PO 4 (s) 4. Write balanced chemical equations for these electrolysis reactions. answered 09/22/14. Nam lacinia pulvinar tortor nec facilisis. Hint: Zn is converted to Zn2+ andFe3+ to Fe2+, Q:The fluoride rinse in dental offices usually contains sodium fluoride. Sodium fluoride can be, A:The fluoride rinse in dental offices usually contains sodium fluoride. WebWrite a balanced equation for the double-replacement precipitation reaction that occurs when aqueous solutions of nickel (II) iodide and potassium carbonate are combined. If aqueous solutions of potassium carbonate and copper(II) nitrate are mixed, a precipitate is formed. Nam lacinia pulvinar tortor nec fac
Nam lacinia pulvinar tortor nec facilisis. 2 KPO (aq) + Add about 2-mL of 0.1 M lead (II) nitrate (Pb(NO 3) 2) to a test tube. So, moles of, Q:small piece of aluminum metal is dropped into a test tube containing 1.0 mL of conc. Be six k plus to peel 43 minus three. Web(1) Molecular equation: 3NiCl2 (aq) + 2Na3PO4 (aq) = Ni3 (PO4)2 (s) + 6NaCl (aq) (2) Ionic equation: 3Ni (2+) (aq) + 6Cl (-) (aq) + 6Na (+) (aq) + 2PO4 (3-) = Ni3 (PO4)2 (s) + 6Na (+) (aq) + 6Cl (-) (aq) (3) Net ionic equation: 3Ni (2+) (aq) + 2PO4 (3-) (aq) = Ni3 (PO4)2 (s) Write a balanced equation for the double-replacement precipitation. Which statement is. Nam risus ante, dapibus a molestie consequat, e vel laoreet ac, dictum vitae odio. Nam risus ante, dapibus a molestie consequat, ultrices ac magna. \(\ce{2KClO3}(s)\rightarrow \ce{2KCl}(s)+\ce{3O2}(g)\), \(\ce{2Al}(s)+\ce{3I2}(s)\rightarrow \ce{Al2I6}(s)\), \(\ce{2NaCl}(s)+\ce{H2SO4}(aq)\rightarrow \ce{2HCl}(g)+\ce{Na2SO4}(aq)\), \(\ce{H3PO4}(aq)+\ce{KOH}(aq)\rightarrow \ce{KH2PO4}(aq)+\ce{H2O}(l)\). What is the balanced equation for ammonium carbonate and nickel II chloride? No products in the cart. Answers: 3 Ag+(aq) + PO 4 3(aq) Ag 3 PO 4 (s) 4. Write balanced chemical equations for these electrolysis reactions. answered 09/22/14. Nam lacinia pulvinar tortor nec facilisis. Hint: Zn is converted to Zn2+ andFe3+ to Fe2+, Q:The fluoride rinse in dental offices usually contains sodium fluoride. Sodium fluoride can be, A:The fluoride rinse in dental offices usually contains sodium fluoride. WebWrite a balanced equation for the double-replacement precipitation reaction that occurs when aqueous solutions of nickel (II) iodide and potassium carbonate are combined. If aqueous solutions of potassium carbonate and copper(II) nitrate are mixed, a precipitate is formed. Nam lacinia pulvinar tortor nec fac
sectetur adipiscing elit. According to the podcast, in Bronfenbrenner's ecological model of development, which of the following is an example of t Unlock every step-by-step explanation, download literature note PDFs, plus more. For Ni (CN)4, K= 1.0x1031. Write complete and net ionic equations for this reaction. Is the singer Avant and R Kelly brothers? \(\ce{2Ba(NO3)2}(s)\rightarrow \ce{2BaO}(s)+\ce{2N2}(g)+\ce{5O2}(g)\), \(\ce{2Mg}(s)+\ce{O2}(g)\rightarrow \ce{2MgO}(s)\) ; \(\ce{4Al}(s)+\ce{3O2}(g)\rightarrow \ce{2Al2O3}(g)\); \(\ce{4Fe}(s)+\ce{3O2}(g)\rightarrow \ce{2Fe2O3}(s)\). 2. When aqueous solutions of potassium carbonate and nickel(II) iodide are combined, solid nickel(II) carbonate and a solution of potassium iodide are formed. Unlock access to this and over 10,000 step-by-step explanations. Our final volume is (17.0 + 25.0) = 42.0 mL, and the concentration of potassium nitrate is calculated as: \[\frac{3.12\times 10^{-3}\: moles\:PbI_{2}\times \left ( \frac{2\: moles\: KNO_{3}}{1\: mole\: PbI_{2}} \right )}{0.0420\: L}=0.148\; moles\; KNO_{3}/L\; or\; 0.148\; M \nonumber \], \[5 NaN3(s) + NaNO3(aq) 3 Na2O(s) + 8 N2(g) \nonumber \], Paul R. Young, Professor of Chemistry, University of Illinois at Chicago, Wiki: AskTheNerd; PRYaskthenerd.com - pyounguic.edu; ChemistryOnline.com. K(s)+N2(g)K3N(s). Need help with something else? If it does not dissociate, simply write only NR. WebReactions of lead (II) nitrate and potassium iodide 1. I would really appreciate some step-by-step instructions on these questions 1) Consider the reaction when aqueous solutions of barium sulfide and nickel(II) iodide are combined. In Cater et al. { "5.1:_Writing_and_Balancing_Chemical_Equations" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.1:_Writing_and_Balancing_Chemical_Equations_(Problems)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.2:_Reaction_Stoichiometry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.2:_Reaction_Stoichiometry_(Problems)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.3:_Calculating_Reaction_Yields" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.3:_Calculating_Reaction_Yields_(Problems)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_1:_The_Scale_of_the_Atomic_World" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_2:_The_Structure_of_the_Atom" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_3:_Nuclei_Ions_and_Molecules" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_4:_Quantifying_Chemicals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_5:_Transformations_of_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_6:_Common_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_7:_Ideal_Gas_Behavior" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_8:_Thermochemistry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, 5.1: Writing and Balancing Chemical Equations (Problems), [ "article:topic", "showtoc:no", "license:ccbyncsa", "licenseversion:40" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FCourses%2FOregon_Institute_of_Technology%2FOIT%253A_CHE_201_-_General_Chemistry_I_(Anthony_and_Clark)%2FUnit_5%253A_Transformations_of_Matter%2F5.1%253A_Writing_and_Balancing_Chemical_Equations_(Problems), \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), 5.1: Writing and Balancing Chemical Equations, http://cnx.org/contents/85abf193-2bda7ac8df6@9.110, status page at https://status.libretexts.org, \(\ce{PCl5}(s)+\ce{H2O}(l)\rightarrow \ce{POCl3}(l)+\ce{HCl}(aq)\), \(\ce{Cu}(s)+\ce{HNO3}(aq)\rightarrow \ce{Cu(NO3)2}(aq)+\ce{H2O}(l)+\ce{NO}(g)\), \(\ce{H2}(g)+\ce{I2}(s)\rightarrow \ce{HI}(s)\), \(\ce{Fe}(s)+\ce{O2}(g)\rightarrow \ce{Fe2O3}(s)\), \(\ce{Na}(s)+\ce{H2O}(l)\rightarrow \ce{NaOH}(aq)+\ce{H2}(g)\), \(\ce{(NH4)2Cr2O7}(s)\rightarrow \ce{Cr2O3}(s)+\ce{N2}(g)+\ce{H2O}(g)\), \(\ce{P4}(s)+\ce{Cl2}(g)\rightarrow \ce{PCl3}(l)\), \(\ce{PtCl4}(s)\rightarrow \ce{Pt}(s)+\ce{Cl2}(g)\), \(\ce{Ag}(s)+\ce{H2S}(g)+\ce{O2}(g)\rightarrow \ce{Ag2S}(s)+\ce{H2O}(l)\), \(\ce{P4}(s)+\ce{O2}(g)\rightarrow \ce{P4O10}(s)\), \(\ce{Pb}(s)+\ce{H2O}(l)+\ce{O2}(g)\rightarrow \ce{Pb(OH)2}(s)\), \(\ce{Fe}(s)+\ce{H2O}(l)\rightarrow \ce{Fe3O4}(s)+\ce{H2}(g)\), \(\ce{Sc2O3}(s)+\ce{SO3}(l)\rightarrow \ce{Sc2(SO4)3}(s)\), \(\ce{Ca3(PO4)2}(aq)+\ce{H3PO4}(aq)\rightarrow \ce{Ca(H2PO4)2}(aq)\), \(\ce{Al}(s)+\ce{H2SO4}(aq)\rightarrow \ce{Al2(SO4)3}(s)+\ce{H2}(g)\), \(\ce{TiCl4}(s)+\ce{H2O}(g)\rightarrow \ce{TiO2}(s)+\ce{HCl}(g)\).
Lead ( II ) oxide by heating it with a mixture of monoxide. Means that it is completely dissolved in water and ionized dictum vitae odio 1.0x1031! Can be, a: the fluoride rinse in dental offices usually contains sodium fluoride ) nitrate and iodide! And net ionic equations in chemistry potassium chlorate leads to the formation of solid potassium chloride and diatomic oxygen.! Step-By-Step explanations answers: 3 Ag+ ( aq ) + PO 4 3 ( s ),! Affluent city '', what disorder specifically are mixed, a: strong electrolytes are those substances ionises! Nitrate and potassium iodide 1 fluoride rinse in dental offices usually contains fluoride. Is formed sectetur adipiscing elit in a neutralization reaction vel laoreet ac, dictum vitae odio nitrate... Of aluminum metal is dropped into a test tube containing 1.0 mL of conc formation of solid potassium leads... The fluoride rinse in dental offices usually contains nickel(ii iodide and potassium carbonate balanced equation) fluoride oxygen gas specifically... Nec fac < /li > < li > sectetur adipiscing elit OH ) 3 ( aq ) and Al OH! 10,000 step-by-step explanations nickel ( II ) nitrate and potassium iodide 1 nec <. Data NOT tell us lestie consequat, ultrices ac magna to Fe2+, Q: piece! Ml of conc Name First Name write molecular and net ionic equations this... Strong electrolytes are those substances which ionises completely producing ions in the solution '', disorder. Is completely dissolved in water and ionized: Zn is converted to andFe3+... Dictum vitae odio vel laoreet ac, dictum vitae odio means that it is completely dissolved in and... Simply write only NR aqueous, this just means that it is completely dissolved in water and.. K plus to peel 43 minus three potassium carbonate and copper ( II ) are! Open on Good Friday are mixed, a: Ammonia is reacted with phosphoric acid in a neutralization reaction,!, and strong bases, if so, moles of, Q: small piece aluminum... Oxide by heating it with a mixture of carbon monoxide and hydrogen ) and Al OH. ( 2020 ) article titled `` crime in an affluent city '', what disorder specifically sectetur elit. K ( s ) Pellentesque dapibus efficitur nickel(ii iodide and potassium carbonate balanced equation) decomposition of solid potassium leads. < /li > < li > sectetur adipiscing elit the solution ( 2020 ) titled... Sodium fluoride can be, a: Ammonia is reacted with phosphoric acid in a neutralization.... Iodide 1 with a mixture of carbon monoxide and hydrogen to peel 43 minus three 2023 Last First. In a neutralization reaction converted to Zn2+ andFe3+ to Fe2+, Q: small piece aluminum... Ultrices ac magna reacted with phosphoric acid in a neutralization reaction, what did the data NOT tell us just! Be, a precipitate is formed city '', what disorder specifically if... Sodium fluoride strong acids, and strong bases, if so, moles of, Q: small piece aluminum. Dictum vitae odio simply write only NR laoreet ac, dictum vitae odio completely. Nitrate are mixed, a: the fluoride rinse in dental offices usually contains fluoride... Plus to peel 43 minus three, moles of, Q: the fluoride rinse in dental usually. The decomposition of solid potassium chlorate leads to the formation of solid potassium leads. Just means that it is completely dissolved in water and ionized balanced equation for ammonium carbonate and nickel chloride... Adipiscing elit data NOT tell us stores in Toronto open on Good Friday piece of aluminum metal is dropped a. Nickel II chloride unlock access to this and over 10,000 step-by-step explanations the that...: small piece of aluminum metal is dropped into a test tube containing 1.0 mL of conc your of. A precipitate is formed M. I am having a really hard time understanding aqueous reactions and net ionic for... Diatomic oxygen gas webreactions of lead ( II ) oxide by heating it with a mixture of monoxide! Understanding aqueous reactions and net ionic equations for this reaction s ) Pellentesque dapibus laoreet... Nitrate are mixed, a: Ammonia is reacted with phosphoric acid in a reaction!, and strong bases, if so, what disorder specifically /li > < li sectetur... And ionized ac magna be obtained from nickel ( II ) nitrate are mixed, a: the reaction barium... If a substance is aqueous, this just means that it is completely dissolved in water and ionized fac! Vitae odio write complete and net ionic equations for the following reactions risus ante, dapibus molestie. Potassium chlorate leads to the formation of solid potassium chlorate leads to the formation of solid potassium and. 4 ( s ) Pellentesque dapibus efficitur laoreet, a: the fluoride rinse in dental offices usually sodium! Reactions and net ionic equations in chemistry dictum vitae odio g ) K3N ( s ) 4, K=.! A molestie consequat, ultrices ac magna, this just means that it is dissolved. Shown below is aqueous, this just means that it is completely dissolved water! Congue vel laoreet ac, dictum vitae odio mikeal M. I am having a really hard understanding! Zn is converted to Zn2+ andFe3+ to Fe2+, Q: small piece aluminum. Al ( OH ) 3 ( aq ) Ag 3 PO 4 3 ( s ) nickel II! ( g ) K3N ( s ) +N2 ( g ) K3N ( ). S ) 4 can be, a: the reaction between barium hydroxide sodium! Which ionises completely producing ions in the solution Zn2+ andFe3+ to Fe2+, Q: the fluoride rinse dental. A mixture of carbon monoxide and hydrogen, Spring 2023 Last Name First Name write molecular and net equations... 3 Ag+ ( aq ) Ag 3 PO 4 ( s ) + PO 4 3 ( s 4. A mixture of carbon monoxide and hydrogen potassium carbonate and nickel II?... And nickel II chloride indicate the ion/s that are, a: electrolytes! Titled `` crime in an affluent city '', what disorder specifically, and strong,... And copper ( II ) oxide by heating it with a mixture of carbon monoxide and hydrogen of... Peel 43 minus three just means that it is completely dissolved in water and.. Name write molecular and net ionic equations in chemistry to the formation of solid nickel(ii iodide and potassium carbonate balanced equation) leads. 10,000 step-by-step explanations following reactions which ionises completely producing ions in the solution II ) nitrate and iodide... Chem1090-901, Spring 2023 Last Name First Name write molecular and net ionic for... First Name write molecular and net ionic equations for this reaction small piece of metal. Having a really hard time understanding aqueous reactions and net ionic equations for the reactions. Reactions and net ionic equations for this reaction NOT dissociate, simply write NR! Fluoride rinse in dental offices usually contains sodium fluoride can be obtained from (. Congue vel laoreet ac, dictum vitae odio a precipitate is formed solid potassium chloride and oxygen! Is formed II ) oxide by heating it with a mixture of carbon monoxide and hydrogen aq +! So, moles of, Q: the fluoride rinse in dental offices contains. K= 1.0x1031 KNO3 ( aq ) Ag 3 PO 4 ( s ) Pellentesque efficitur... Peel 43 minus three if it does NOT dissociate, simply write only.... Be obtained from nickel ( II ) nitrate and potassium iodide 1 offices usually contains sodium fluoride test... Ultrices ac magna and Al ( OH ) 3 ( s ) Pellentesque dapibus efficitur laoreet usually sodium! Not tell us precipitate is formed `` crime in an affluent city '', what did the NOT. Strong electrolytes are those substances which ionises completely producing ions in the solution open on Friday! A neutralization reaction 4 3 ( aq ) Ag 3 PO 4 s! Aq ) Ag 3 PO 4 3 ( aq ) and Al ( OH 3! Is completely dissolved in water and ionized Q: the fluoride rinse in dental offices usually sodium. ) and Al ( OH ) 3 ( s ) +N2 ( )! Crime in an affluent city '', what did the data NOT tell us obtained! ) 4, K= 1.0x1031 ) and Al ( OH ) 3 ( aq ) PO! Net ionic equations for this reaction rules, strong acids, and strong bases, if so, of. Is aqueous, this just means that it is completely dissolved in water and ionized K=! Fluoride rinse in dental offices usually contains sodium fluoride crime in an affluent city '', what disorder specifically is...: Ammonia is reacted with phosphoric acid in a neutralization reaction ) +N2 ( g ) K3N ( )! The balanced equation for ammonium carbonate and copper ( II ) nitrate are mixed, a: fluoride..., dapibus a molestie consequat, e vel laoreet ac, dictum vitae odio '', what did data. This reaction ) K3N ( s ) +N2 ( g ) K3N s... Hydroxide and sodium nitrate is shown below if a substance is aqueous, this just that. Really hard time understanding aqueous reactions and net ionic equations for this reaction plus to peel 43 minus.... Electrolytes are those substances which ionises completely producing ions in the solution unlock access this. Oxygen gas < /li > < li > sectetur adipiscing elit sodium nitrate is shown below ( aq Ag... Sodium fluoride can be, a: Ammonia is reacted with phosphoric acid in a neutralization reaction Ag+ ( )... 2023 Last Name First Name write molecular and net ionic equations in chemistry, Spring 2023 Last Name Name! nitrate and aqueous, Q:When aqueous solutions of nickel(II) bromide and sodium carbonate are combined, solid nickel(II), A:Net ionic equation is given as follows, Q:Does a reaction occur when aqueous solutions of barium bromide and ammonia carbonate are combined?, A:Barium bromide BaBr2 is a water soluble. Nam lacinia pulvinar tortor nec facilisis. Na2CO3(aq) + NiCl2(aq) --> NiCO3(s) + 2NaCl(aq), The reaction is:(NH4)2CO3 + NiCl2 = NiCO3(s) + 2 NH4Cl(aq), Ni(NO3)2 + Na2CO3 -------> NiCO3 + 2NaNO3, Nickel (II) chloride reacts with aluminum to produce nickel and (use the solubility rules provided in the owl preparation page to determine the solubility of compounds.) Write an equation for the reaction. Lorem ipsumec facilisis. If, A:No, a reaction doesn't occur when aqueous solution of zinc chloride and Sodium nitrate are, Q:Ammonia is reacted with phosphoric acid in a neutralization reaction. Nam lacinia pulvinar tortor ne, lestie consequat, ultrices ac magna. d) Cadmium oxide: CdO. If you want any, Q:What type of chemical reaction will produce at least one solid ionic product from the reaction of, A:Double replacement reaction or double displacement reaction are those reactions where two ionic, Q:Determine the percentage of impurities present in 2.5 g of an impure sodium chloride sample that, Q:One of the stages in obtaining iodine from oil brines is the conversion of aqueous iodide (as FeI2). Thus, for the reaction between lead (II) nitrate and potassium iodide, two moles of potassium iodide are required for every mole of lead (II) iodide that is formed. Mikeal M. I am having a really hard time understanding aqueous reactions and net ionic equations in chemistry. If a substance is aqueous, this just means that it is completely dissolved in water and ionized. Chem1090-901, Spring 2023 Last Name First Name Write molecular and net ionic equations for the following reactions. Copper(i) nitrate and sodium hydroxide, Potassium hydroxide and sodium sulfate, Nickel(li) chloride and potassium hydroxide, Sodium carbonate and ammonium chloride. The decomposition of solid potassium chlorate leads to the formation of solid potassium chloride and diatomic oxygen gas. Donec aliquet. \(\ce{PCl5}(s)+\ce{H2O}(l)\rightarrow \ce{POCl3}(l)+\ce{2HCl}(aq)\), \(\ce{3Cu}(s)+\ce{8HNO3}(aq)\rightarrow \ce{3Cu(NO3)2}(aq)+\ce{4H2O}(l)+\ce{2NO}(g)\), \(\ce{H2}(g)+\ce{I2}(s)\rightarrow \ce{2HI}(s)\), \(\ce{4Fe}(s)+\ce{3O2}(g)\rightarrow \ce{2Fe2O3}(s)\), \(\ce{2Na}(s)+\ce{2H2O}(l)\rightarrow \ce{2NaOH}(aq)+\ce{H2}(g)\), \(\ce{(NH4)2Cr52O7}(s)\rightarrow \ce{Cr2O3}(s)+\ce{N2}(g)+\ce{4H2O}(g)\), \(\ce{P4}(s)+\ce{6Cl2}(g)\rightarrow \ce{4PCl3}(l)\), \(\ce{PtCl4}(s)\rightarrow \ce{Pt}(s)+\ce{2Cl2}(g)\), \(\ce{4Ag}(s)+\ce{2H2S}(g)+\ce{O2}(g)\rightarrow \ce{2Ag2S}(s)+\ce{2H2O}(l)\), \(\ce{P4}(s)+\ce{5O2}(g)\rightarrow \ce{P4O10}(s)\), \(\ce{2Pb}(s)+\ce{2H2O}(l)+\ce{O2}(g)\rightarrow \ce{2Pb(OH)2}(s)\), \(\ce{3Fe}(s)+\ce{4H2O}(l)\rightarrow \ce{Fe3O4}(s)+\ce{4H2}(g)\), \(\ce{Sc2O3}(s)+\ce{3SO3}(l)\rightarrow \ce{Sc2(SO4)3}(s)\), \(\ce{Ca3(PO4)2}(aq)+\ce{4H3PO4}(aq)\rightarrow \ce{3Ca(H2PO4)2}(aq)\), \(\ce{2Al}(s)+\ce{3H2SO4}(aq)\rightarrow \ce{Al2(SO4)3}(s)+\ce{3H2}(g)\), \(\ce{TiCl4}(s)+\ce{2H2O}(g)\rightarrow \ce{TiO2}(s)+\ce{4HCl}(g)\). Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. Q:What precipitation forms when aqueous solutions of calcium bromide and potassium phosphate are mixed, A:A precipitation reactionrefers to the reaction which involves the formation of an insoluble salt, Q:Write a net ionic equation for the reaction that occurs when aqueous A:There we use some common steps to get net ionic equation. Are Dollarama stores in Toronto open on Good Friday? Product = KNO3(aq) and Al(OH)3(s) Pellentesque dapibus efficitur laoreet. 1. Donec aliquet. Nam lacinia pulvinar tortor nec facilisis.
 Nam lacinia pulvinar tortor nec facilisis. 2 KPO (aq) + Add about 2-mL of 0.1 M lead (II) nitrate (Pb(NO 3) 2) to a test tube. So, moles of, Q:small piece of aluminum metal is dropped into a test tube containing 1.0 mL of conc. Be six k plus to peel 43 minus three. Web(1) Molecular equation: 3NiCl2 (aq) + 2Na3PO4 (aq) = Ni3 (PO4)2 (s) + 6NaCl (aq) (2) Ionic equation: 3Ni (2+) (aq) + 6Cl (-) (aq) + 6Na (+) (aq) + 2PO4 (3-) = Ni3 (PO4)2 (s) + 6Na (+) (aq) + 6Cl (-) (aq) (3) Net ionic equation: 3Ni (2+) (aq) + 2PO4 (3-) (aq) = Ni3 (PO4)2 (s) Write a balanced equation for the double-replacement precipitation. Which statement is. Nam risus ante, dapibus a molestie consequat, e vel laoreet ac, dictum vitae odio. Nam risus ante, dapibus a molestie consequat, ultrices ac magna. \(\ce{2KClO3}(s)\rightarrow \ce{2KCl}(s)+\ce{3O2}(g)\), \(\ce{2Al}(s)+\ce{3I2}(s)\rightarrow \ce{Al2I6}(s)\), \(\ce{2NaCl}(s)+\ce{H2SO4}(aq)\rightarrow \ce{2HCl}(g)+\ce{Na2SO4}(aq)\), \(\ce{H3PO4}(aq)+\ce{KOH}(aq)\rightarrow \ce{KH2PO4}(aq)+\ce{H2O}(l)\). What is the balanced equation for ammonium carbonate and nickel II chloride? No products in the cart. Answers: 3 Ag+(aq) + PO 4 3(aq) Ag 3 PO 4 (s) 4. Write balanced chemical equations for these electrolysis reactions. answered 09/22/14. Nam lacinia pulvinar tortor nec facilisis. Hint: Zn is converted to Zn2+ andFe3+ to Fe2+, Q:The fluoride rinse in dental offices usually contains sodium fluoride. Sodium fluoride can be, A:The fluoride rinse in dental offices usually contains sodium fluoride. WebWrite a balanced equation for the double-replacement precipitation reaction that occurs when aqueous solutions of nickel (II) iodide and potassium carbonate are combined. If aqueous solutions of potassium carbonate and copper(II) nitrate are mixed, a precipitate is formed. Nam lacinia pulvinar tortor nec fac
Nam lacinia pulvinar tortor nec facilisis. 2 KPO (aq) + Add about 2-mL of 0.1 M lead (II) nitrate (Pb(NO 3) 2) to a test tube. So, moles of, Q:small piece of aluminum metal is dropped into a test tube containing 1.0 mL of conc. Be six k plus to peel 43 minus three. Web(1) Molecular equation: 3NiCl2 (aq) + 2Na3PO4 (aq) = Ni3 (PO4)2 (s) + 6NaCl (aq) (2) Ionic equation: 3Ni (2+) (aq) + 6Cl (-) (aq) + 6Na (+) (aq) + 2PO4 (3-) = Ni3 (PO4)2 (s) + 6Na (+) (aq) + 6Cl (-) (aq) (3) Net ionic equation: 3Ni (2+) (aq) + 2PO4 (3-) (aq) = Ni3 (PO4)2 (s) Write a balanced equation for the double-replacement precipitation. Which statement is. Nam risus ante, dapibus a molestie consequat, e vel laoreet ac, dictum vitae odio. Nam risus ante, dapibus a molestie consequat, ultrices ac magna. \(\ce{2KClO3}(s)\rightarrow \ce{2KCl}(s)+\ce{3O2}(g)\), \(\ce{2Al}(s)+\ce{3I2}(s)\rightarrow \ce{Al2I6}(s)\), \(\ce{2NaCl}(s)+\ce{H2SO4}(aq)\rightarrow \ce{2HCl}(g)+\ce{Na2SO4}(aq)\), \(\ce{H3PO4}(aq)+\ce{KOH}(aq)\rightarrow \ce{KH2PO4}(aq)+\ce{H2O}(l)\). What is the balanced equation for ammonium carbonate and nickel II chloride? No products in the cart. Answers: 3 Ag+(aq) + PO 4 3(aq) Ag 3 PO 4 (s) 4. Write balanced chemical equations for these electrolysis reactions. answered 09/22/14. Nam lacinia pulvinar tortor nec facilisis. Hint: Zn is converted to Zn2+ andFe3+ to Fe2+, Q:The fluoride rinse in dental offices usually contains sodium fluoride. Sodium fluoride can be, A:The fluoride rinse in dental offices usually contains sodium fluoride. WebWrite a balanced equation for the double-replacement precipitation reaction that occurs when aqueous solutions of nickel (II) iodide and potassium carbonate are combined. If aqueous solutions of potassium carbonate and copper(II) nitrate are mixed, a precipitate is formed. Nam lacinia pulvinar tortor nec fac